25-27 kDa (Light Chain) & 53-55 kDa (Heavy Chain) (Reducing)
Reconstitute at 0.1-1 mg/ml according to the size in ultrapure water after rapid centrifugation.
1. Matthew Krautmann. Laboratory safety evaluation of lokivetmab, a canine anti-interleukin-31 monoclonal antibody, in dogs. Vet Immunol Immunopathol. 2023 Apr:258:110574.
Lokivetmab is a caninized anti-IL-31 mAb that binds and neutralizes canine IL-31, resulting in reduced skin lesions and inhibition of pruritus in dogs with AD or allergic dermatitis. Lokivetmab is the first mAb approved for use in dogs with canine AD and allergic dermatitis by regulatory agencies worldwide. Lokivetmab targets canine IL-31 specifically and does not bind to murine or human IL-31 or to any other canine proteins. After s.c. administration, lokivetmab is absorbed and incorporated into the pool of circulating antibodies. The long terminal elimination half-life of lokivetmab in dogs of 16.5 ± 3.0 days is due to recycling via the FcRn receptor and allows for monthly injections to maintain efficacy. Elimination by catabolism is identical to any endogenous protein. Lokivetmab binds at a single epitope on canine IL-31, forming a mAb:IL-31 complex having pharmacokinetics and elimination like unbound lokivetmab.
Measured by its binding ability in a functional ELISA. When Lokivetmab(Anti-Canine IL-31Recombinant Antibody) is immobilized 0.5µg/mL (100µL/well), Recombinant Canine IL-31 binds with an EC50 of 0.07-1ng/ml.
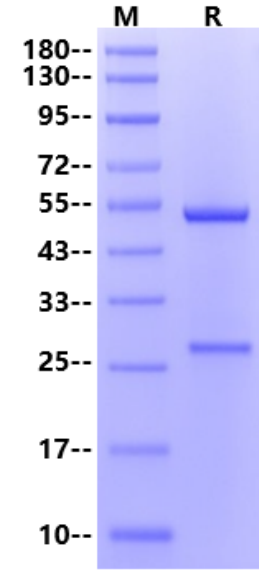
2μg (R: reducing condition).