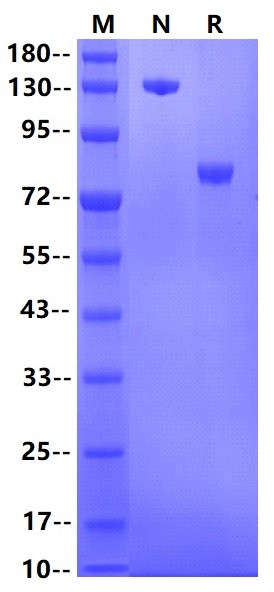
Siglec-8, also known as SAF, is an approximately 75 kDa transmembrane glycoprotein in the Siglec family of sialic acid-binding immune regulatory molecules . Mature human Siglec-8 consists of a 347 amino acid (aa) extracellular domain (ECD) with three Ig-like domains, a 21 aa transmembrane segment, and a 115 aa cytoplasmic domain with two tyrosine based signaling motifs . Alternative splicing generates additional isoforms that either lack most of the second Ig-like domain or have a substituted cytoplasmic domain without the signaling motifs . Siglec-8 is expressed on eosinophils, basophils, and mast cells , and it shows a binding preference for the carbohydrate 6-O sulfated sLex . In mouse, Siglec-F is a functional paralog of human Siglec-8, as shown by its similar expression profile and ligand specificity . Cross-linking of Siglec-8 inhibits Fc epsilon RI alpha induced mast cell degranulation . It also induces eosinophil apoptosis, an effect which is enhanced by the eosinophil-activating cytokines IL-5, IL-33, and GM-CSF .