Leu33-His287, with C-terminal 8*His
LECYSCVQKADDGCSPHRMKTVKCGPGVDVCTEAVGAVETIHGQFSVAVRGCGSGIPGKNDRGLDLHGLLAFFQLQQCSEDRCNAKLNLTLRGLNPAGNESAYEPNGAECYSCVGLSREKCQGSMPPVVNCYNASGRVYKGCFDGNVTLTAANVTVSLPVRGCVQDETCTRDGVTGPGFTLSGSCCQGPRCNADLRNKTYFSPRIPPLVLLPPPTTAAPSTRAQNSSSTTSTAAPTTTTSIIKPTTAQASHTSPHGGGSHHHHHHHH
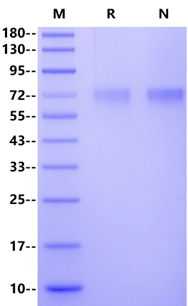
>95% by SDS-PAGE
LYPD3 (Ly6/PLAUR domain-containing protein 3, C4.4A), first reported in 1998, is a tumorigenic and high-glycosylated cell surface protein that has been proven to be linked with the carcinogenic effects in different solid tumors. The extracellular region of LYPD3 includes two such LU domains (D1 and D2) followed by a C-terminal region, which is rich in serine, threonine and proline. Circumstantial evidence suggests that LYPD3 plays a role in adhesion, migration and invasion similar to that of uPAR. Aberrant expression of LYPD3 plays an oncogenic role in several types of cancer. Expression of the human LYPD3 was observed by RT-PCR and Northern blotting in placental tissue, skin, esophagus and peripheral blood leukocytes, but not in brain, lung, liver, kidney, stomach, colon and lymphoid organs. As demonstrated for malignant melanoma, LYPD3 mRNA expression correlated with tumor progression. The elevated expression of LYPD3 is not only demonstrated to be associated with lung adenocarcinoma carcinogenesis and poor prognosis but also there is evidence that LYPD3 can lead to the initiation and development of cancers and the chemoresistance of metastatic cancers by impacting the and apoptosis of the tumor, which are involved in many important regulatory mechanisms of cancers. This suggests LYPD3 as a potential diagnostic marker. In addition, LYPD3 might serve as a therapeutic target. First preclinical results of a phase I study with a LYPD3-directed antibody-drug conjugate in non-small cell lung cancer showed a sufficient antitumor efficacy in in vivo models.