Val 26-Leu204, with C-terminal Human IgG1 Fc
VPQKPKVSLNPPWNRIFKGENVTLTCNGNNFFEVSSTKWFHNGSLSEETNSSLNIVNAKFEDSGEYKCQHQQVNESEPVYLEVFSDWLLLQASAEVVMEGQPLFLRCHGWRNWDVYKVIYYKDGEALKYWYENHNISITNATVEDSGTYYCTGKVWQLDYESEPLNITVIKAPREKYWLGGGSGGGSPKSSDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
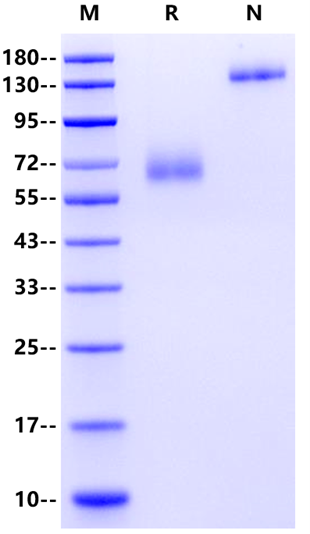
>95% by SDS-PAGE
Fc epsilon RI, the high-affinity IgE receptor, distinguishes Ag-IgE interactions and drives cellular allergic responses. Fc epsilon RI is primarily expressed on MCs, basophils and Ag-presenting cells, and mainly exists as the heterotetramer αβγ2. However, there are differences among species: an alternate trimeric form αγ2 is expressed on human, but not rodent. Structurally, it is composed of one α-chain bearing two extracellular immunoglobulin domains that bind to the Fc portion of a single molecule of IgE, a β-chain that holds an immunoreceptor tyrosine-based activation motif (ITAM), and a homodimer of disulfide-bound ITAM-containing γ-chains. Expression of the β chain in mast cells and basophils results in increased Fc epsilon RI surface expression and amplifies signaling through the receptor. The importance of the α-subunit in the Fc epsilon RI-mediated allergic reaction was demonstrated by the absence of allergic reactions in α-subunit-deficient mice. The Fc epsilon RIα binds to the Fc fragment of IgE at a 1:1 ratio to form the IgE-Fc complex. Fc epsilon RI is a unique molecular target that initiates different functional outcomes of MC responses and allergic inflammation. The recognition of multivalent antigen by Fc epsilon RI-bound IgE triggers a complex biochemical pathway initiated by the phosphorylation of ITAM motifs by the Src family kinase Lyn. Fc epsilon RI not occupied by IgE has a half-life on the mast cell surface of 24 hours in vitro, while receptors bound to IgE appear to be expressed for the life of the cell. The density of human basophil FcεR1 expression correlates directly with serum IgE levels, where binding of IgE stabilizes the receptor at the cell surface.
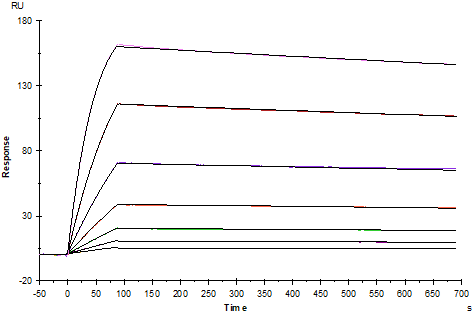
Protein A Chip captured Fc epsilon RI α Fc Chimera, Human (Cat. No. UA010147), can bind Human IgE Protein with an affinity constant of 0.12nM as determined in SPR assay.

Immobilized Fc epsilon RI α Fc Chimera, Human (Cat. No. UA010147) at 0.5μg/mL (100μL/well) can bind Human IGHE His Tag with EC50 of 0.88-1.40 ng/mL.