Ser33-Gly167, with C-terminal Human IgG1 Fc
SGHASSTPGGEKETSATQRSSVPSSTEKNAFNSSLEDPSTDYYQELQRDISEMFLQIYKQGGFLGLSNIKFRPGSVVVQLTLAFREGTINVHDVETQFNQYKTEAASRYNLTISDVSVSDVPFPFSAQSGAGVPGIEGRMDPKSSDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
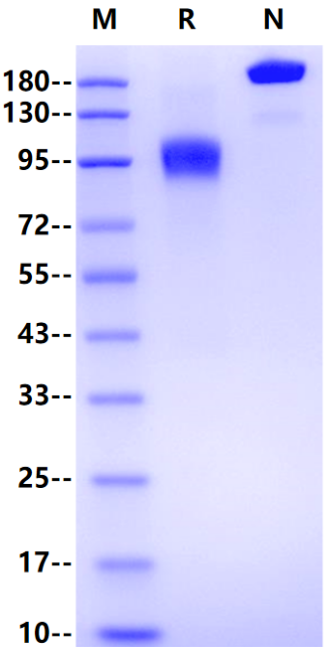
>95% by SDS-PAGE
Mucin 1 (MUC1) is a highly glycosylated transmembrane protein expressed mainly on the apical surface of most glandular epithelial cells, including those of the mammary gland, lung, pancreas, kidney, female reproductive tract and stomach. It is also expressed on MM cell lines, fresh patient MM cells, and plasmacytomas, as well as other hematological malignancies, including Ki-1-positive B-cell lymphomas, T-cell lymphomas, Hodgkin's disease, and malignant histiocytosis. MUC1 functions primarily in the formation of a physical barrier to lubricate and protect normal epithelial tissues and mediate signal transduction. However, aberrant expression of MUC1 occurs in cancer cells, including those of esophageal cancer, gastric cancer, breast cancer, ovarian cancer, bladder cancer and other tumors, and is especially prominent in breast cancer cells. The biological structure of MUC1 consists of both a C-terminal fragment (MUC1-C) and an N-terminal fragment (MUC1-N) linked by non-covalent interactions. Furthermore, the C-terminal fragment comprises an extracellular region, a transmembrane region and an intracellular region. Among these regions, the transmembrane region (28 amino acids) and intracellular region (72 amino acids) are highly conserved. The N-terminal fragment is located on the membrane surface and contains the signal peptide, the (30–90) variable number tandem repeat (VNTR) region, and the sea urchin sperm protein, enterokinase and agrin (SEA) domain. Aberrantly glycosylated MUC1 is associated with cellular transformation from a normal to malignant phenotype in human cancers. Furthermore, Muc-1 binds to intercellular adhesion molecule-1 (ICAM-1), suggesting a role in cellular migration.