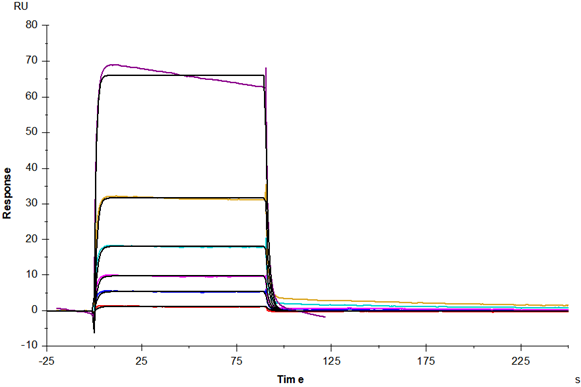
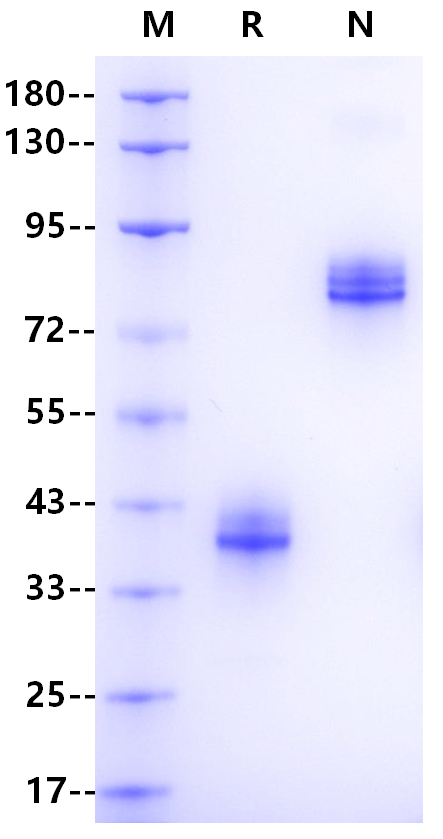
Immunoglobulin G (IgG) is a monomer immunoglobulin, mainly involved in secondary antibody reaction, plasma B cell synthesis and secretion, constitute 75% of human serum immunoglobulin. There are four subtypes of IgG antibody: IgG1, IgG2, IgG3 and IgG4, which decrease in turn in serum. The spatial structure of these four subtypes is similar and the heavy chain sequences of each subtype are highly homologous. IgG3 has an extended hinge region, and its core hinge region has 11 disulfide bonds, so it is unstable for protease cleavage. After pepsin cleavage, IgG3 is divided into two antigen binding sites F (ab) s and a highly conserved Fc segment, and the Fc segment has a highly conserved N-glycosylation site. Fc fragments have been shown to mediate phagocytosis, trigger inflammation, and target IgG to specific tissues. The binding ability of IgG3 to Fc γ Rs was the strongest, and the effects of ADCC, ADCP and CDC were stronger than that of IgG1.