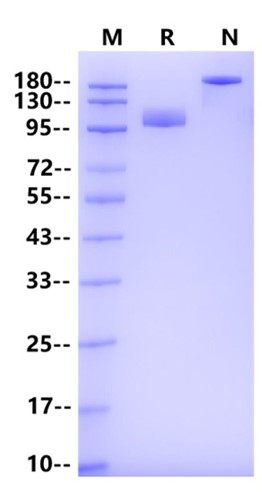
>95% by SDS-PAGE
Siglec-4, also known as myelin-associated glycoprotein (MAG) is a 100 kDa glycoprotein located at the innermost layer of myelin sheets that remains in intimate contact with the axonal membrane. It is selectively expressed by myelinating cells including Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS). MAG, a member of the sialic binding, immunoglobulin-like lectin (siglec) family, is a critical component of axon-glia interactions with multiple functions in the biology of both neurons and glial cells. A membrane-bound protein, MAG contains five extracellular immunoglobulin-like domains, and the NH2-terminal V-type immunoglobulin domain also contains the lectin domain. Known ligands for MAG include both proteins and glycolipids. It functions both as a ligand for an axonal receptor that is needed for the maintenance of myelinated axons and as a receptor for an axonal signal that promotes the differentiation, maintenance and survival of oligodendrocytes. MAG is expressed as two developmentally regulated isoforms with different cytoplasmic domains that may activate different signal transduction pathways in myelin-forming cells.