28 kDa (Reducing)
>98% by SDS-PAGE & RP-HPLC
20mM Tris-HCl, pH8.0, 150mM NaCl, 1mM EDTA, 5mM DTT, 50%(v/v) Glycerol
· 12 months from date of receipt, -20 to -70 °C as supplied.
· 6 months, -20 to -70 °C under sterile conditions after reconstitution.
· 1 week, 2 to 8 °C under sterile conditions after reconstitution.
· Please avoid repeated freeze-thaw cycles.
The tobacco etch virus (TEV) protease is a useful tool for the removal of fusion tags from recombinant proteins. TEV protease has a strict 7 amino acid cleavage recognition sequence of Glu-Asn-Leu-Tyr-Phe-Gln-Gly/Ser [ENLYFQ(G/S)] and cleavage occurs between the Gln and Gly/Ser residues, The most commonly used sequence is ENLYFQG. It is recommended that the cleavage for each fusion protein be optimized by varying the amount of recombinant viral TEV protease, reaction time, or incubation temperature. It can be removed by Ni2+ affinity resin. Recombinant Tobacco Etch Virus Protease (rTEV) has (NIa) protease catalytic domain which corresponds to a molecular weight of 28 kDa. It is unique with high specificity and is active at low temperature. rTEV Protease has a 6*His-tag for easy removal from a reaction using nickel affinity resins and has been engineered to improve thermal stability and decrease autolysis.
1. Add the following to a microcentrifuge tube:Add the following to a microcentrifuge tube:
Fusion Protein | 15μg |
10X rTEV Protease Buffer +/– Salt
| 5μl |
rTEV Protease
|
5U
|
ddH2O
|
To 50μl |
2. Mix and incubate at 30°C, Remove 10 μL aliquots at 1, 2, 4, and 6 hours.
3. Analyze by SDS-PAGE.
Guidelines for Cleavage
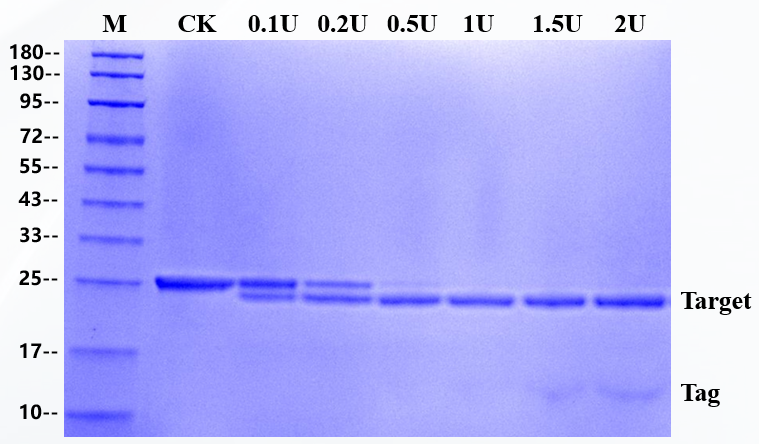
The substrate of 3 μg fusion protein was digested by enzyme, and the addition amount of rTEV Protease in the sample was 0.1U, 0.2U, 0.5U, 1U,1.5U and 2U, respectively. The substrate is a fusion protein with a molecular weight of 25 KD and reacted at 30 ℃ for 1 hour, and the target band of 23kD is produced after rTEV Protease digestion.

