N.A
62kDa
25 mM Tris-acetate (pH7.8),1 mM EDTA, 1 mM DTT, 50% glycerol, 0.2 M ammonium sulfate
N.A
12 months from date of receipt, -20 to -70 °C as supplied; 6 months, -20 to -70 °C under sterile conditions after reconstitution; 1 week, 2 to 8 °C under sterile conditions after reconstitution; Please avoid repeated freeze-thaw cycles.
1.Mcelroy H H S D .THE COLORS OF FIREFLY BIOLUMINESCENCE: ENZYME CONFIGURATION AND SPECIES SPECIFICITY[J].Proceedings of the National Academy of Sciences of the United States of America, 1964, 52(1):75-81.
2.Conti E , Franks N P , Brick P .Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes[J].Structure, 1996, 4(3):287.
Luciferase is a general term for enzymes that produce biofluorescence in nature. Luciferase can catalyze the oxidation of luciferin to oxyluciferin. In the process of luciferin oxidation, biofluorescence is emitted. The biofluorescence released during the oxidation of luciferin can then be measured by a fluorometer. The Luciferase came from the firefly Photinus pyralis (Ppy) catalyzes a two-step reaction that results in the oxidation of D-luciferin accompanied by emission of yellow−green light with a peak at 560 nm. However, wild-luciferase activity is inhibited by sodium chloride, Therefore, the amino acid mutation we performed improved the inhibition of sodium chloride and was eventually named Luciferase Ⅰ.
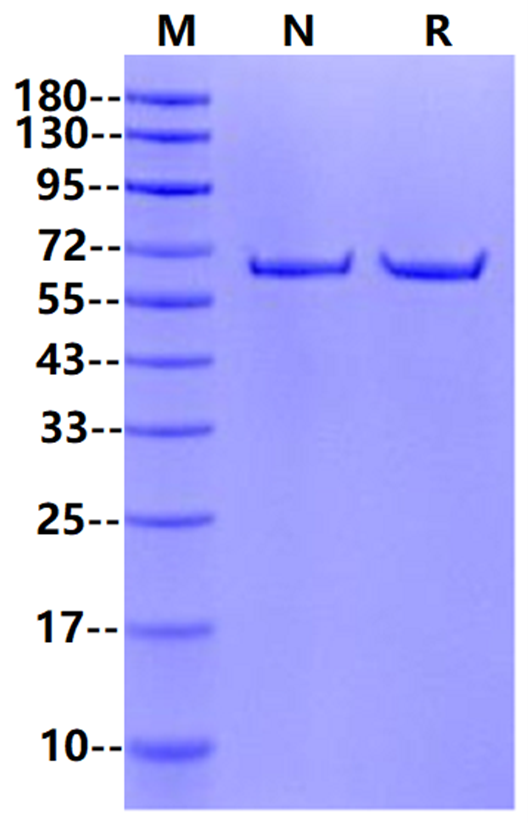
M: marker ; Complement Luciferase Ⅰ,2μg on SDS-PAGE under reducing and Non-reducing condition. The purity is greater than 95%.