Leu23-Gly150, with C-terminal Human IgG1 Fc LSVQQGPNLLQVRQGSQATLVCQVDQATAWERLRVKWTKDGAILCQPYITNGSLSLGVCGPQGRLSWQAPSHLTLQLDPVSLNHSGAYVCWAAVEIPELEEAEGNITRLFVDPDDPTQNRNRIASFPGIEGRMDPKSSDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
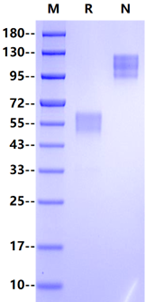
>95% by SDS-PAGE
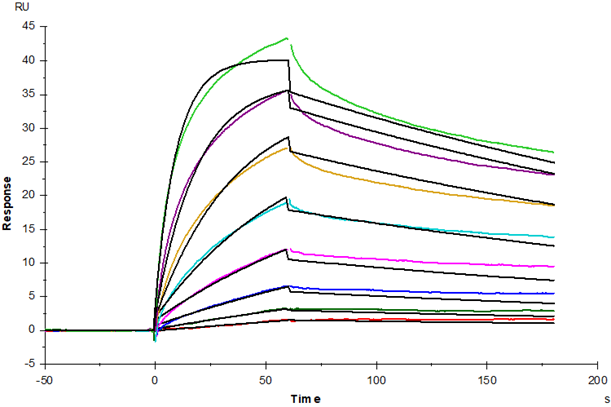
Transmembrane and immunoglobulin domain containing 2 (TMIGD2)is new a immune checkpoint molecule of the B7:CD28 family which is a novel cell adhesion receptor that is expressed in various human organs and tissues, mainly in cells with epithelium and endothelium origins. TMIGD2 regulates cellular morphology, homophilic cell aggregation, and cell-cell interaction. TMIGD2 activity also modulates actin stress fiber formation and focal adhesion and reduces cell migration. Silencing of expression of TMIGD2 by small interfering RNA (siRNA) and by ectopic overexpression in endothelial cells showed that TMIGD2 regulates capillary tube formation in vitro, and B16F melanoma cells engineered to express TMIGD2 displayed extensive angiogenesis in the mouse Matrigel angiogenesis model. Moreover, TMIGD2, through its proline-rich cytoplasmic domain, associates with multiple Src homology 3 (SH3)-containing signaling proteins, including SH3 protein interacting with Nck (SPIN90/WISH), bullous pemphigoid antigen-1, and calcium channel β2.Silencing of expression of SPIN90/WISH by siRNA in endothelial cells showed that SPIN90/WISH is required for capillary tube formation. These features of TMIGD2 suggest that TMIGD2 is a novel receptor that plays an important role in cell-cell interaction, cell migration, and angiogenesis.