Glu22-Val202, with C-terminal 6*His
EPPTQKPKKIVNAKKDVVNTKMFEELKSRLDTLAQEVALLKEQQALQTVCLKGTKVHMKCFLAFTQTKTFHEASEDCISRGGTLGTPQTGSENDALYEYLRQSVGNEAEIWLGLNDMAAEGTWVDMTGARIAYKNWETEITAQPDGGKTENCAVLSGAANGKWFDKRCRDQLPYICQFGIVGGGSHHHHHH
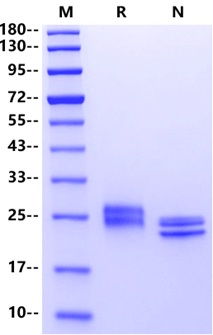
>95% by SDS-PAGE&RP-HPLC
C-type lectin domain family 3 member B (CLEC3B) is a hexameric, multimodular extracellular matrix protein with several molecular forms that are created through alternative splicing and protein modifications. It is highly conserved amongst vertebrates, and molecular phylogeny indicating that it evolved before fibronectin. CLEC3B has many extracellular binding partners, including matrix components, soluble factors and pathogens; it also influences cell phenotype directly through interactions with cell surface receptors. The synthesis of CLEC3B is strictly regulated, and it is widely distributed in embryonic tissues while restricted in adult tissues. CLEC3B is also de novo expressed in wound healing or pathological state, including chronic inflammation and cancer. First described as a modulator of cell adhesion, CLEC3B also directs a plethora of cell signaling and gene expression programs by shaping mechanical and biochemical cues in the cellular microenvironment. Exploitation of the pathological expression and function of CLEC3B is emerging as a promising strategy to develop new diagnostic, therapeutic and bioengineering tools.