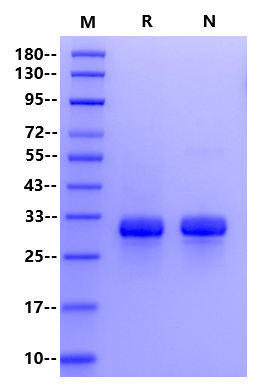
>95% by SDS-PAGE
Angiopoietin-1 (Ang-1) is a secreted glycoprotein that plays a critical role in the development and maintenance of the vascular system. It contains a N-terminal coiled-coil region and a C-terminal fibrinogen-like domain separated by a short flexible region. Angiopoietin-1 binds and activates the receptor tyrosine kinase Tie-2, and its association into tetramers is important for full Tie-2 activation. Angiopoietin-1 ligation of Tie-2 on vascular endothelial cells (EC) induces the development and branching of blood vessels. In confluent EC (i.e. in homeostasis), multimeric Angiopoietin-1 enhances vascular integrity by promoting the in trans homotypic association of Tie-2 between EC or with the substratum. In addition, Angiopoietin-1 suppresses several VEGF-induced effects on the vasculature including endothelial permeability, stretch-induced release of Angiopoietin-2, and up-regulation of the leukocyte adhesion molecules VCAM-1, ICAM-1, and E-Selectin. Angiopoietin-1 also interacts with a variety of integrins and the extracellular matrix independently of Tie-2. These interactions support the adhesion, migration and stress resistance of EC, fibroblasts, and myocytes. Angiopoietin-1 can protect against pulmonary arterial hypertension, reduce the extent of fibrosis and remodeling in infarcted diabetic myocardium, and enhance tumor progression and metastasis.