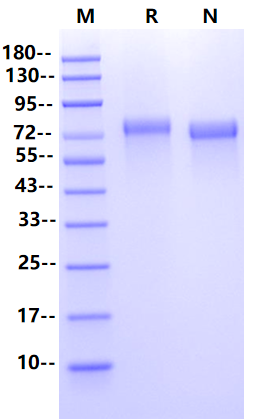
Glu22-Ala397, with C-terminal 8*His
EHPTADRAGCSASGACYSLHHATMKRQAAEEACILRGGALSTVRAGAELRAVLALLRAGPGPGGGSKDLLFWVALERRRSHCTLENEPLRGFSWLSSDPGGLESDTLQWVEEPQRSCTARRCAVLQATGGVEPAGWKEMRCHLRANGYLCKYQFEVLCPAPRPGAASNLSYRAPFQLHSAALDFSPPGTEVSALCRGQLPISVTCIADEIGARWDKLSGDVLCPCPGRYLRAGKCAELPNCLDDLGGFACECATGFELGKDGRSCVTSGEGQPTLGGTGVPTRRPPATATSPVPQRTWPIRVDEKLGETPLVPEQDNSVTSIPEIPRWGSQSTMSTLQMSLQAESKATITPSGSVISKFNSTTSSATPQAFDSSSAHHHHHHHH
C-type lectin domain family 14 member A (CLEC14A), also known as epidermal growth factor receptor 5 (EGFR-5), is an approximately 52-kDa member of the C-type lectin domain family that plays a role in angiogenic functions including cell-cell adhesion, endothelial cell migration, and tube formation. This tumor endothelial marker is a type I transmembrane protein whose extracellular domain (ECD) domain consists of a 141 amino acid (aa) C-type lectin domain, a 43 aa EGF-like domain, and a sushi-like domain. Within the ECD, human CLEC14A shares 67% and 68% sequence identity with mouse and rat CLEC14A, respectively. In endothelial cells, CLEC14A forms a complex with VEGFR-3. Failure in the formation of this complex has been observed to result in the enhanced expression and activity of VEGFR-2 and the downstream signaling. By its interaction with VEGFR-3, CLECA4 appears to regulate developmental angiogenesis, lymphangiogenesis, and pathological angiogensis. CLEC14A acts in vascular homeostasis by fine-tuning VEGF R2 and VEGF R3 signaling in ECs, suggesting its relevance in the pathogenesis of angiogenesis-related human disorders.