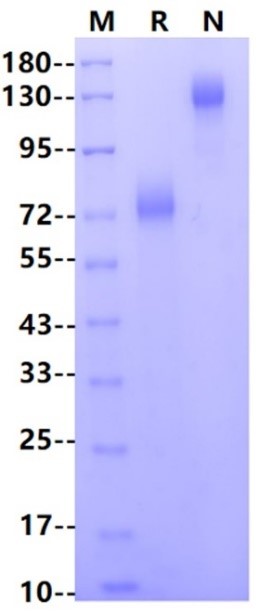
Asp20-Arg687, with C-terminal Human IgG Fc
125-130 kDa (Reducing)
PBS, pH7.4
Reconstitute at less than 1 mg/mL according to the size in ultrapure water after rapid centrifugation .
· 12 months from date of receipt, lyophilized powder stored at -20 to -80℃.
· 3 months, -20 to -80℃ under sterile conditions after reconstitution.
· 1 week, 2 to 8℃ under sterile conditions after reconstitution.
· Please avoid repeated freeze-thaw cycles.
Sialic acid-binding immunoglobulin-like lectins (Siglecs) are type I immunoglobulin-like transmembrane proteins consisting of an extracellular structural domain, a transmembrane structural domain, and an intracellular structural domain. The intracellular domain is divided into a short lysine-containing tail and an extracellular structural domain consisting of an N-terminal binding Ig domain and a variable number of C2-type structural domains. Siglec-2 (CD22) belongs to a family of immune inhibitory Siglecs, which bears ITIM to deliver immunosuppressive signals such as reduced phagocytosis, dampened inflammation, and inhibited danger-associated molecular pattern/ pathogen-associated molecular pattern (DAMP/PAMP)-mediated inflammation. Siglec-2 is a cell surface receptor expressed mostly on B cells that regulates B-cell proliferation, survival, signaling, and antibody production. The structure of Siglec-2 contains six tyrosines in its cytoplasmic tail, four of which are in ITIM motifs: Y783, Y843, Y863, and Y828. Following cross-linking of B-cell receptors (BCRs), these tyrosine residues are phosphorylated and recruit Src homology region 2 (SH2) domain-containing protein tyrosine phosphatase-1 (SHP-1), which dephosphorylates BCR-proximal signaling complexes. Siglec-2 is an attractive therapeutic target considering its unique presence in B lymphocytes. Currently, many ongoing trials evaluating CD22-directed chimeric antigen receptors (CARs), particularly in children with relapsed or refractory B-cell leukemia, are showing safety and efficacy. Some novel CARs, such as bispecific CD20/CD22 CARs and CD19/CD22 CARs, are also in development.
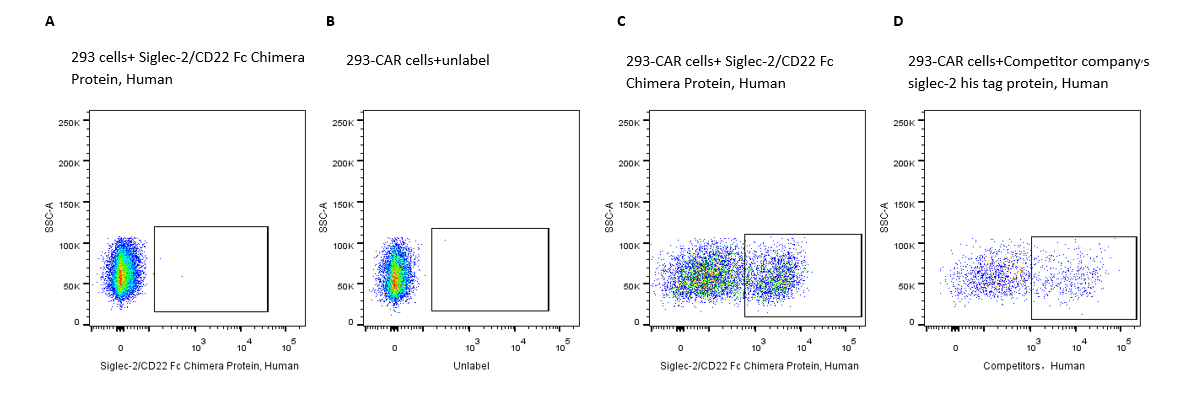
2e5 of transient transfected anti-Siglec-2 ScFv CAR-293 cells were stained with 0.1ug Siglec-2/CD22 Fc Chimera Protein, Human, (Cat. No. UA010025) and unlabel respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). Alexa Fluor® 647 signal was used to evaluate the binding activity.
2e5 of transient transfected anti-Siglec-2 ScFv CAR-293 cells were stained with competitor respectively (Fig. D). APC signal was used to evaluate the binding activity.
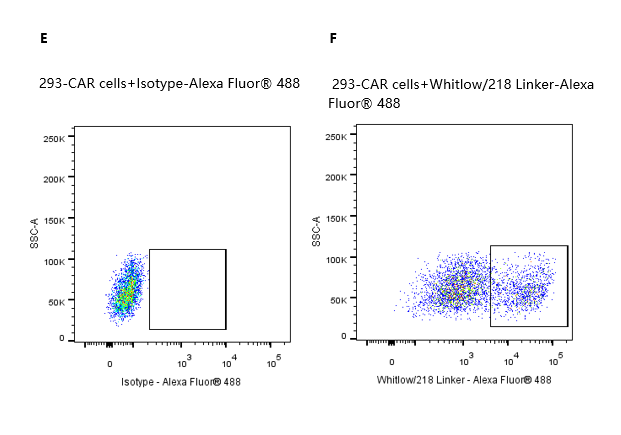
2e5 of transient transfected anti-Siglec-2 ScFv CAR-293 cells were stained with isotype and Whitlow/218 Linker-Alexa Fluor® 488 (Fig. E and F). Alexa Fluor® 488 signal was used to evaluate the binding activity.

Immobilized Siglec-2/CD22 Fc Chimera, Human (Cat. No. UA010025) at 2.0μg/mL (100μL/well) can bind Biotinylated Anti-Human CD22 (Pinatuzumab) with EC50 of 4.32-6.15ng/mL.