Asp155-Ala709, with C-terminal 10*His DWVIPPISCPENEKGPFPKNLVQIKSNKDKEGKVFYSITGQGADTPPVGVFIIERETGWLKVTEPLDRERIATYTLFSHAVSSNGNAVEDPMEILITVTDQNDNKPEFTQEVFKGSVMEGALPGTSVMEVTATDADDDVNTYNAAIAYTILSQDPELPDKNMFTINRNTGVISVVTTGLDRESFPTYTLVVQAADLQGEGLSTTATAVITVTDTNDNPPIFNPTTYKGQVPENEANVVITTLKVTDADAPNTPAWEAVYTILNDDGGQFVVTTNPVNNDGILKTAKGLDFEAKQQYILHVAVTNVVPFEVSLTTSTATVTVDVLDVNEAPIFVPPEKRVEVSEDFGVGQEITSYTAQEPDTFMEQKITYRIWRDTANWLEINPDTGAISTRAELDREDFEHVKNSTYTALIIATDNGSPVATGTGTLLLILSDVNDNAPIPEPRTIFFCERNPKPQVINIIDADLPPNTSPFTAELTHGASANWTIQYNDPTQESIILKPKMALEVGDYKINLKLMDNQNKDQVTTLEVSVCDCEGAAGVCRKAQPVEAGLQIPAGGGSGGGSHHHHHHHHHH
1. Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008 Apr;36(Pt 2):149-55.
2. Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000 Feb 7;148(3):399-404.
CDH1 (also known as epithelial cadherin (E-cadherin), a member of the cadherin- catenin complex, is an example in which pathogenic variants can lead to CLP and/or gastric cancer. The human CDH1 gene consists of 16 exons that span 100 kb. Classical cadherins are a family of single-span transmembrane domain glycoproteins that primarily mediate calcium-dependent cell–cell adhesion, which are differentially expressed throughout the body. Cadherins are single-membrane-spanning proteins with a divergent extracellular domain of five repeats and a conserved cytoplasmic domain. Much experimental evidence suggests that the CDH1 is a tumor suppressor and alterations in its expression are linked with multiple tumor types and its clinicopathological features. Loss of CDH1 is used for the diagnosis and prognosis of epithelial cancers.
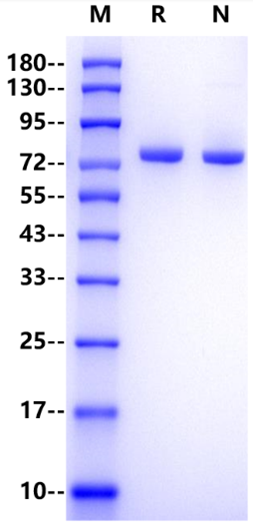
1μg (R: reducing conditions, N: non-reducing conditions).