Leu18-Ser214, with C-terminal Human IgG Fc LCNSDGSQSLHMLQISYFQDNHHVRHQGNASLGKLLTHTLEGPSQNVTILQLQPWQDPESWERTESGLQIYLTQFESLVKLVYRERKENVFFPLTVSCSLGCELPEEEEEGSEPHVFFDVAVNGSAFVSFRPKTAVWVSGSQEPSKAANFTLKQLNAYNRTRYELQEFLQDTCVEFLENHITTQNMKGSQTGRSYTSIEGRMDPKSSDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
1. Montes, R. et al. (2012) Thromb. Haemost. 107:815. 2. Bae, J.-S. et al. (2007) Blood 110:3909. 3. Fink, K. et al. (2013) PLoS One 8:e53103. 4. Willcox, C.R. et al. (2012) Nat. Immunol. 13:872. 5. Turner, L. et al. (2013) Nature 498:502.
The endothelial protein C receptor (EPCR), also known as CD201, is an approximately 50 kDa transmembrane glycoprotein expressed on vascular endothelial cells and functions as a negative regulator of thrombosis. EPCR inhibits thrombosis through its interactions with Protein C, activated Protein C (APC), and Coagulation Factors VII, and VIIa. It enhances the activation of Protein C in response to complexes of Thrombin-Thrombomodulin. In humans, a soluble form of EPCR can be produced by alternative splicing or ADAM17/TACE mediated shedding, and this protein inhibits the anti-coagulant activity of APC. EPCR can be degraded on the surface of endothelial cells by Neutrophil Elastase. Activation of EPCR also protects vascular endothelial cells from Thrombin-induced apoptosis.EPCR binds to CD11b/CD18 (Mac-1) on monocytes and mediates monocyte adhesion to the vascular endothelium. In addition, EPCR binds to the antigen receptor on gamma δ T cells, promotes hematopoietic stem cell retention in the bone marrow, and binds to surface proteins of some species of Plasmodium, contributing to pathogenicity in severe malaria.
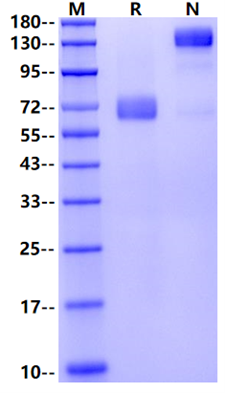
1μg (R: reducing condition, N: non-reducing condition).