27 kDa (Reducing)
1 mM HCl, 2 mM CaCl2, pH 3.0
Reconstitute at 0.1-1 mg/ml according to the size in ultrapure water after rapid centrifugation.
Store at -25 ~ -15℃ for 2 years
1. Simon LM, Kotormán M, Garab G, Laczkó I. Structure and activity of alpha-chymotrypsin and trypsin in aqueous organic media. Biochem Biophys Res Commun. 2001 Feb 9;280(5):1367-71. PMID: 11162681.
2. Pham VT, Ewing E, Kaplan H, Choma C, Hefford MA. Glycation improves the thermostability of trypsin and chymotrypsin. Biotechnol Bioeng. 2008 Oct 15;101(3):452-9. PMID: 18470893.
Chymotrypsin is a serine endoproteinase that specifically cleaves peptide bonds at the C-termini of Tyr, Phe, Trp and Leu. Met, Ala, Asp and Glu may be cleaved at a much lower rate.
Recombinant α-Chymotrypsin, human lyophilized powder
Optimal incubation times and enzyme concentrations must be determined empirically for a particular substrate. Typical reaction conditions are as follows:
Add Recombinant α-Chymotrypsin, human, to a final protease: protein ratio of 1:200 to 1:20(w/w), and incubate sample for 2–18 hours at 25°C. The reaction may be stopped, if desired, by adding 0.5% trifluoroacetic acid.
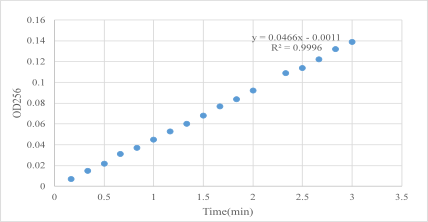
In the experimental design, BTEE substrate was prepared, and 6 ul α-chymotrypsin of 0.2 mg/mL was added to the uartz cuvette with an optical path of 1cm of 2994 ul substrate and the absorption value of 256 nm was read every 10 seconds at 25 ℃.
1μg (R: reducing condition, N: non-reducing condition).
98.7%