24 kD
12 month, 2 to 8 °C under sterile conditions
Store at -20℃ and avoid repeated freeze-thaw cycles.
[1] Charles M , Rovery M , Guidoni A ,et al.Trypsinogen and trypsin of pig[J].Biochimica et Biophysica Acta, 1963, 69:115-129.
[2] Keryn,Dallas,Johnson,et al.A functional comparison of ovine and porcine trypsins[J].Comparative Biochemistry & Physiology Part B Biochemistry & Molecular Biology, 2002.
Trypsin (EC3.4.21.4) is part of the serine protease family. Trypsin cleaves lysine and arginine at the C-terminal side of the peptide. The hydrolysis rate is slower if an acidic residue is on either sides of the cleavage site and no cleavage occurs if a proline residue is on the carboxyl side of the cleavage site. The stringent specificity of trypsin is essential for protein identification, and it has become the gold standard for protein digestion to peptides for shotgun proteomics. Trypsin optimum pH is pH-7 to 9. Trypsin will also hydrolyze ester and amide linkages of synthetic derivatives of amino acids such as: benzoyl L-arginine ethyl ester (BAEE), p-toluenesulfonyl- L-arginine methyl ester (TAME), tosyl-L-arginine methyl ester, N-α-benzoyl-L-arginine p-nitroanilide (BAPNA), L-lysyl-p-nitroanilide, and benzoyl-L-tyrosine ethyl ester (BTEE). Serine protease inhibitors that inhibit recombinant trypsin include TLCK (N-p-tosyl-L-lysine chloromethyl ketone), PMSF (phenylmethanesulfonyl fluoride), benzamidine, soybean trypsin inhibitor, and ovomucoid.
1x, 0.15 g Recombinant porcine trypsin and 0.02 g EDTA per liter of 0.9% sodium chloride
Trypsin Solution may be used to remove adherent cells from a culture surface.
1. Remove medium from culture vessel by aspiration and wash the monolayer with PBS to remove all traces of serum. Remove salt solution by aspiration.
2. Dispense enough Trypsin Solution into culture vessel(s) to completely cover the monolayer of cells and place in RT or 37℃ incubator for 2-3 minutes.
3. Remove the Trypsin Solution by aspiration and return closed culture vessel(s) to incubator. The coated cells are allowed to incubate until cells detach from the surface. Progress can be checked by examination with an inverted microscope.
4.When trypsinization process is complete the cells will be in suspension and appear rounded.
5. It is advisable to add serum or medium containing serum to the cell suspension as soon as possible to inhibit further tryptic activity which may damage cells.
6. Cells can be resuspended by gently pipetting the cell suspension to break up the clumps. Further dilution can be made, if required, for cell counts and/or subculturing.
The time required to remove cells from the culture surface is dependent on cell type, population density, serum concentration in the growth medium, potency of trypsin, and time since last subculture. Trypsin can cause cellular damage, thus time of exposure should be kept to a minimum.

In the experimental design, 0.25 mM BAEE substrate was prepared, and 10 ul Trypsin of 0.54 mg/mL was added to the uartz cuvette with an optical path of 1cm of 3000 ul substrate and 190 ul buffer, and the absorption value of 253 nm was read every 30 seconds at 25 ℃.
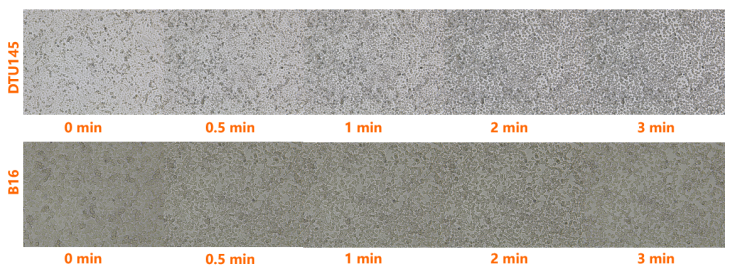
Dispense enough Trypsin Solution into culture vessel(s) to completely cover the monolayer of cells and place in RT incubator for 3 minutes.
2μg (R: reducing condition).